If you’re looking for an answer to the question, “Which compound is a tertiary alcohol?” you’ve come to the right place. In this article, we’ll discuss the properties of a tertiary alcohol and explore the various compounds that can be classified as such. We’ll also look at the chemical reaction that occurs during the production of a tertiary alcohol and discuss its potential uses. By the end of this article, you’ll have a better understanding of tertiary alcohols and be able to identify them in a variety of compounds.
A tertiary alcohol is an organic compound in which the hydroxy group, -OH, is attached to a saturated carbon atom. Examples of tertiary alcohols include 2-methyl-2-butanol and tert-amyl alcohol (TAA). TAA is an isomer of n-amyl alcohol and is commonly used as a solvent in various applications. It is also a precursor for other compounds such as esters, amides, and ethers.
Contents
- What is a Tertiary Alcohol?
- Properties of Tertiary Alcohols
- Which Compound is a Tertiary Alcohol?
- Top 6 Frequently Asked Questions
- What is a Tertiary Alcohol?
- What is the Difference Between Primary, Secondary and Tertiary Alcohols?
- What is a Compound?
- Which Compound is a Tertiary Alcohol?
- How are Tertiary Alcohols Used?
- What are the Hazards of Tertiary Alcohols?
- Primary, Secondary, and Tertiary Alcohols: Classification, Examples, & Practice
What is a Tertiary Alcohol?
A tertiary alcohol is an organic compound that contains an alcoholic hydroxyl group (-OH) attached to a saturated carbon atom. This type of alcohol is also known as a tertiary hydroxyalkane or an aliphatic alcohol. It is an important class of compounds in organic chemistry and is widely used in the production of pharmaceuticals, fragrances, and other industrial chemicals.
Tertiary alcohols have a higher boiling point than primary and secondary alcohols due to the increased number of hydrogen bonds formed between the molecules. This makes them ideal for use in applications that require a higher boiling point. They are also more resistant to oxidation than primary and secondary alcohols, making them useful for applications that require a longer shelf life.
Properties of Tertiary Alcohols
Tertiary alcohols have the general molecular formula of CnH2n+1OH. They are characterized by having a single alcohol group attached to a saturated carbon atom. This makes them more stable than primary and secondary alcohols, which have two or more alcohol groups attached to a saturated carbon atom.
Tertiary alcohols are generally more soluble in water than primary or secondary alcohols. This property makes them useful for applications that require a higher solubility. They also have a higher boiling point than primary and secondary alcohols due to the increased number of hydrogen bonds formed between the molecules.
Synthesis of Tertiary Alcohols
Tertiary alcohols can be synthesized using a variety of methods, including the Williamson ether synthesis, the Barton-McCombie deoxygenation, and the Williamson-Hay synthesis. Each of these methods involves the use of a different reagent to convert an alkyl halide into a tertiary alcohol.
The Williamson ether synthesis involves the reaction of an alkyl halide with a sodium alkoxide, which produces a tertiary alcohol. The Barton-McCombie deoxygenation is a two-step reaction involving the use of a strong acid such as sulfuric acid and a strong base such as sodium hydroxide to remove oxygen from an alcohol molecule. The Williamson-Hay synthesis is a three-step reaction that involves the use of a strong base, a strong acid, and an alkyl halide to form a tertiary alcohol.
Applications of Tertiary Alcohols
Tertiary alcohols are widely used in the production of pharmaceuticals, fragrances, and other industrial chemicals. They are commonly used as solvents and lubricants due to their high solubility in water and their high boiling point. They are also used as starting materials for the syntheses of other compounds such as esters, ketones, and amines.
Tertiary alcohols are also used in the production of detergents, cleaners, and emulsifiers. They are also used as a preservative in food products and as a fuel additive in gasoline. In addition, they are used to manufacture paints, dyes, and other industrial chemicals.
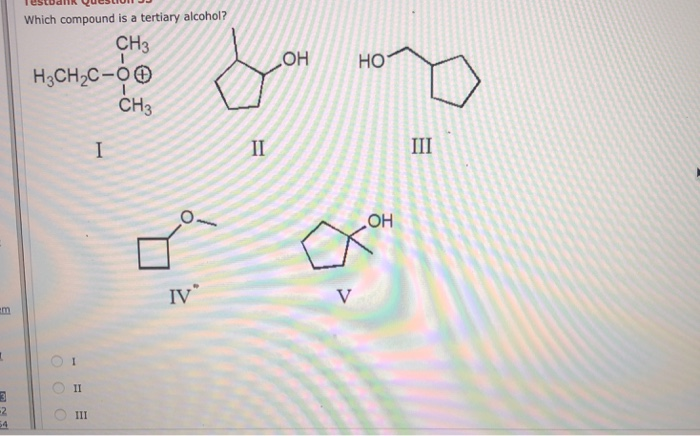
Which Compound is a Tertiary Alcohol?
Tertiary alcohols are organic compounds that contain an alcoholic hydroxyl group (-OH) attached to a saturated carbon atom. They can be identified by their general molecular formula of CnH2n+1OH, as well as their higher boiling point and increased solubility in water compared to primary and secondary alcohols.
Examples of Tertiary Alcohols
There are many examples of tertiary alcohols, such as isopropyl alcohol (2-propanol), butanol (2-butanol), tert-butanol (2-methyl-2-propanol), and tert-amyl alcohol (2-methyl-2-butanol). These compounds are widely used in the production of pharmaceuticals, fragrances, and other industrial chemicals.
Conclusion
Tertiary alcohols are organic compounds that contain an alcoholic hydroxyl group (-OH) attached to a saturated carbon atom. They are characterized by their general molecular formula of CnH2n+1OH, their higher boiling point and increased solubility in water compared to primary and secondary alcohols. Examples of tertiary alcohols include isopropyl alcohol, butanol, tert-butanol, and tert-amyl alcohol. These compounds are widely used in the production of pharmaceuticals, fragrances, and other industrial chemicals.
Top 6 Frequently Asked Questions
What is a Tertiary Alcohol?
A tertiary alcohol is an organic compound that has three carbon atoms attached to the hydroxyl group (OH). The carbon atoms connected to the OH group are called alkyl groups. These alkyl groups can be the same or different. Tertiary alcohols are generally more stable than primary and secondary alcohols due to the presence of additional alkyl groups. They are also more resistant to oxidation, but are more difficult to synthesize.
What is the Difference Between Primary, Secondary and Tertiary Alcohols?
Primary alcohols have one alkyl group attached to the OH group, secondary alcohols have two alkyl groups, and tertiary alcohols have three alkyl groups. The additional alkyl groups in tertiary alcohols make them more stable and resistant to oxidation than primary and secondary alcohols.
What is a Compound?
A compound is a substance composed of two or more elements in a fixed ratio. Compounds can be organic or inorganic and can exist as solids, liquids, or gases. Compounds are held together by chemical bonds, which are formed when atoms share or transfer electrons.
Which Compound is a Tertiary Alcohol?
A number of compounds can be classified as tertiary alcohols. Examples include 2-methyl-2-butanol, 2-methyl-1-propanol, 2,2-dimethyl-1-propanol, and 2,2-dimethyl-2-propanol.
How are Tertiary Alcohols Used?
Tertiary alcohols are used in a variety of applications. They are used as solvents, fuel additives, and intermediates in the production of other compounds. They are also used in the manufacture of detergents and as catalysts in organic reactions.
What are the Hazards of Tertiary Alcohols?
Tertiary alcohols can be hazardous when handled or used incorrectly. They are highly flammable and can cause skin and eye irritation. Inhalation of vapors can cause nose and throat irritation and difficulty breathing. Tertiary alcohols should be handled with caution and care and protective gear should be worn when handling them.
Primary, Secondary, and Tertiary Alcohols: Classification, Examples, & Practice
In conclusion, tertiary alcohols are unique compounds that have a wide range of uses in various industries. They are formed when an alcohol functional group is bonded to three carbon-hydrogen groups and can be used as solvents, fuel additives, and pharmaceuticals. Understanding the difference between primary, secondary, and tertiary alcohols is important for those in the scientific field, as the correct compound must be used for any given task.

