The boiling point of any substance is important to know, especially for those in industries such as chemistry, engineering and food processing. Ethyl alcohol, also known as ethanol, is a common type of alcohol used in a variety of products, ranging from alcoholic beverages to fuel. Knowing the normal boiling point of ethyl alcohol can help to determine the right temperature to be used in various applications. In this article, we will discuss what the normal boiling point of ethyl alcohol is and why it is important.
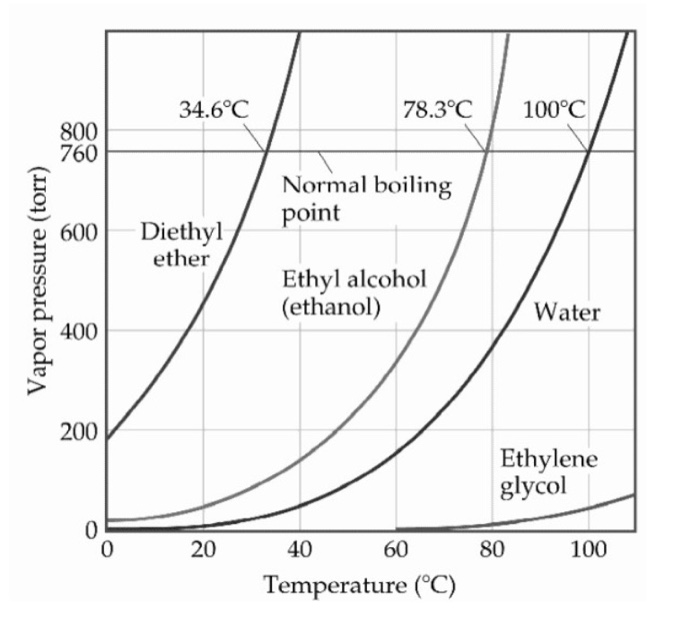
The normal boiling point of ethyl alcohol (ethanol) is 78.37°C (172.9°F). Ethyl alcohol is a colorless, volatile, flammable liquid with a boiling point slightly higher than that of water. It has a wide range of uses, including in fuel, solvents, pharmaceuticals, and as a preservative. It is also used to manufacture a variety of products such as paint, varnish, and antifreeze.
What is the Normal Boiling Point of Ethyl Alcohol?
Ethyl alcohol, also known as ethanol, is a flammable, colorless chemical compound with a distinct odor. It is commonly found in alcoholic beverages and is also used in a variety of industrial and medical applications. The normal boiling point of ethyl alcohol is 78.37°C (173.07°F).
In order to understand the normal boiling point of ethyl alcohol, it is important to first understand the concept of boiling points. A boiling point is the temperature at which a liquid begins to vaporize or boil. At this temperature, the vapor pressure of the liquid is equal to the atmospheric pressure. Different substances have different boiling points, depending on the type of intermolecular forces present between their molecules.
The Intermolecular Forces of Ethyl Alcohol
The intermolecular forces of ethyl alcohol are hydrogen bonds and London forces. Hydrogen bonds are the strongest type of intermolecular force and occur when a hydrogen atom is covalently bonded to a highly electronegative atom like oxygen or nitrogen. London forces are the weaker type of intermolecular forces that occur between non-polar molecules due to temporary dipoles formed by the motion of electrons.
The presence of both hydrogen bonds and London forces in ethyl alcohol allow for a relatively high boiling point compared to other alcohols. For example, methanol, which only has London forces between its molecules, has a boiling point of 65°C (149°F).
Factors that Affect the Boiling Point of Ethyl Alcohol
The boiling point of ethyl alcohol is affected by a variety of factors, including the atmospheric pressure, the presence of impurities, and the concentration of the alcohol. As the atmospheric pressure increases, the boiling point of the alcohol increases. Impurities and higher concentrations of the alcohol can also increase the boiling point.
At higher altitudes, where the atmospheric pressure is lower, the boiling point of ethyl alcohol is lower. For example, at an altitude of 2000 meters, the boiling point of ethyl alcohol is 75.5°C (167.9°F).
Uses of Ethyl Alcohol
Ethyl alcohol is used in a variety of industrial, medical, and recreational applications. It is used as a fuel, a solvent, and an antiseptic. It is also used in the production of perfumes, medicines, and alcohol-based beverages.
In addition to its industrial and medicinal uses, ethyl alcohol is also consumed recreationally as an alcoholic beverage. Beer, wine, and spirits are all commonly consumed alcoholic beverages that contain ethyl alcohol.
Safety of Ethyl Alcohol
Despite its many uses, ethyl alcohol is highly flammable and should be handled with care. It should not be ingested in large quantities as it can be toxic and cause severe health problems. It should also not be used as a fuel for internal combustion engines or other combustible materials.
In addition, ethyl alcohol is a volatile substance and should be stored in a cool, dry place away from sources of ignition. It should also be kept out of the reach of children and animals.
Top 6 Frequently Asked Questions
What is the chemical formula of ethyl alcohol?
The chemical formula of ethyl alcohol is C2H5OH. Ethyl alcohol, also known as ethanol, is a colorless, flammable liquid that is the active ingredient in alcoholic beverages. It is an organic compound composed of two carbon atoms, six hydrogen atoms, and one oxygen atom. Ethanol is produced by fermentation of sugars and other carbohydrates or can be synthesized from petroleum products.
What is the molar mass of ethyl alcohol?
The molar mass of ethyl alcohol is 46.07 g/mol. The molar mass is calculated by adding together the atomic weights of the constituent atoms. In the case of ethanol, this is 2 x 12 g/mol (for the two carbon atoms) plus 6 x 1 g/mol (for the six hydrogen atoms) plus 1 x 16 g/mol (for the oxygen atom), resulting in a total of 46.07 g/mol.
What is the normal boiling point of ethyl alcohol?
The normal boiling point of ethyl alcohol is 78.37°C (172.67°F). The boiling point is the temperature at which the vapor pressure of a liquid is equal to the atmospheric pressure. Above this temperature, the vapor pressure of the liquid is great enough to form bubbles which can be seen in the liquid.
What is the vapor pressure of ethyl alcohol at its normal boiling point?
At its normal boiling point of 78.37°C (172.67°F), the vapor pressure of ethyl alcohol is 1.014 bar. Vapor pressure is the pressure exerted by a vapor or gas when it is in equilibrium with its liquid or solid form. It is a measure of the tendency of the vapor to escape from the liquid or solid.
What is the density of ethyl alcohol?
The density of ethyl alcohol is 0.789 g/ml (at 20°C). Density is a measure of the mass of a substance per unit of volume. Ethyl alcohol is a liquid and its density is affected by temperature; its density increases slightly as the temperature is lowered.
What is the solubility of ethyl alcohol in water?
The solubility of ethyl alcohol in water is approximately 1.2 g/100 mL (at 25°C). Solubility is the ability of a substance to dissolve in a solvent and is usually expressed as the mass of the substance per unit volume of the solvent. Ethyl alcohol is miscible with water, meaning it can be completely dissolved in water.
In conclusion, the normal boiling point of ethyl alcohol is 78.37 degrees Celsius or 173.1 degrees Fahrenheit. This is the temperature at which this particular type of alcohol turns from a liquid to a gas. Understanding the boiling point of ethyl alcohol is important for any professional working in a laboratory, as it helps them to safely handle the substance.

