Generic drugs are revolutionizing the healthcare industry. They offer the same quality and effectiveness of brand-name medications at a fraction of the cost. But what exactly is a generic drug and how does it differ from a brand-name drug? In this article, we explore the world of generic drugs and the potential savings they offer to consumers.
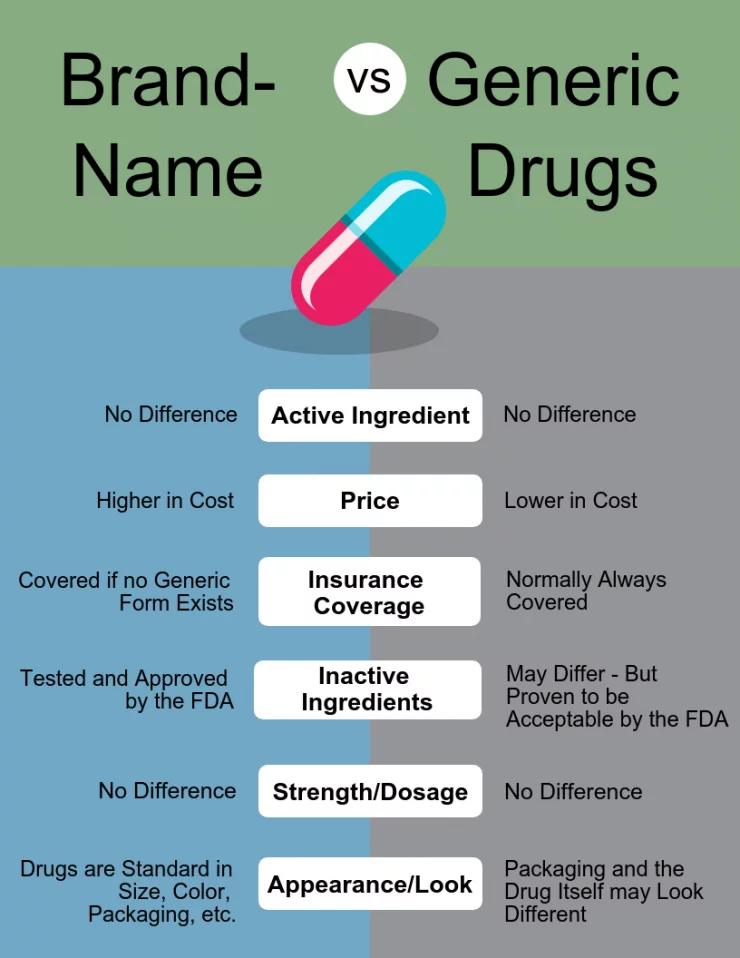
A generic drug is a medication created to be the same as an already marketed brand-name drug in dosage form, safety, strength, route of administration, quality, and performance characteristics. A generic drug is identical, or bioequivalent, to a brand name drug in dosage form, safety, strength, route of administration, quality, performance and intended use. Generic drugs use the same active ingredients as brand-name drugs and work the same way. The FDA requires generic drugs to have the same quality, strength, and purity as brand-name drugs.
What are Generic Drugs?
Generic drugs are identical to name-brand drugs in terms of dosage, safety, strength, quality, route of administration, performance characteristics, and intended use. Generic drugs are typically less expensive than their brand-name counterparts. They are also referred to as generic equivalents or generic medicines. Generic drugs are approved by the US Food and Drug Administration (FDA) and are required to meet the same standards of safety, effectiveness, and quality as brand-name drugs.
Generic drugs are made by manufacturers who don’t have to duplicate the expensive clinical trials of the brand-name drugs. The generic manufacturers must demonstrate to the FDA that their drug is the same as a brand-name drug in terms of active ingredients, route of administration, strength, quality, and performance characteristics.
Generic drugs are typically sold at a lower cost than their brand-name equivalents, as the manufacturers of generic drugs don’t have to bear the costs of developing and marketing a new drug. Generic drugs are also available in the same dosage forms and strengths as the brand-name drug, which allows pharmacists to substitute generic products for brand-name drugs.
Types of Generic Drugs
There are two types of generic drugs: single-source and multi-source. Single-source generic drugs are copies of a brand-name drug that are made by one manufacturer, whereas multi-source generic drugs are copies of a brand-name drug made by multiple manufacturers.
Single-source generic drugs are typically more expensive than multi-source generic drugs, as the manufacturer who produces the single-source drug has the exclusive rights to market the drug and is the only manufacturer who can make it.
Multi-source generic drugs are typically less expensive than single-source generic drugs, as the manufacturers who produce the multi-source drugs don’t have the exclusive rights to market the drug and there can be multiple manufacturers making the same drug.
How are Generic Drugs Regulated?
Generic drugs are regulated by the US Food and Drug Administration (FDA). The FDA is responsible for ensuring that generic drugs meet the same standards of safety, effectiveness, and quality as brand-name drugs.
The FDA has established a set of regulations and standards for generic drugs to ensure that they are safe and effective. Generic drugs must be approved by the FDA before they can be marketed. The FDA also conducts regular inspections of generic drug manufacturers to ensure that the drugs they produce meet the same standards of safety, effectiveness, and quality as brand-name drugs.
Advantages of Generic Drugs
Generic drugs can provide a cost-effective alternative to brand-name drugs, as they are typically less expensive. They also provide an effective treatment option for many health conditions. Generic drugs are also subject to the same standards of safety, effectiveness, and quality as brand-name drugs, ensuring that patients receive a safe and effective treatment option.
Disadvantages of Generic Drugs
Generic drugs may not always be available in the same dosage forms and strengths as brand-name drugs. In addition, some generic drugs may not have the same inactive ingredients as brand-name drugs, which can cause allergies or other adverse reactions in some patients.
Safety of Generic Drugs
Generic drugs are subject to the same standards of safety, effectiveness, and quality as brand-name drugs. Generic drugs must be approved by the US Food and Drug Administration (FDA) and must meet the same safety and quality standards as brand-name drugs.
Conclusion
Generic drugs are an effective and cost-effective alternative to brand-name drugs. They are subject to the same standards of safety, effectiveness, and quality as brand-name drugs, and are typically less expensive.
Few Frequently Asked Questions
What is a Generic Drug?
A generic drug is a pharmaceutical drug that is equivalent to a brand-name product in dosage, strength, route of administration, quality, performance, and intended use. Generic drugs are typically less expensive than the brand-name equivalents.
How Are Generic Drugs Approved?
Generic drugs are approved by the U.S. Food and Drug Administration (FDA) through its Abbreviated New Drug Application (ANDA) process. This process requires generic drug manufacturers to demonstrate that their product is equivalent to an existing approved brand-name drug. The FDA also requires generic drug manufacturers to demonstrate that their processes and manufacturing facilities meet quality and safety standards.
What Are the Benefits of Generic Drugs?
Generic drugs provide many benefits to patients. They are typically much less expensive than brand-name drugs, which can help to improve access to medications for those who are unable to afford the more expensive brand-name products. Generic drugs can also help to reduce health care costs for individuals and insurers. Additionally, generic drugs can help to increase the availability of medications, which can benefit patients by providing more options for treatment.
Are Generic Drugs Safe and Effective?
Yes, generic drugs are safe and effective. They must meet the same standards of safety, quality, and efficacy as brand-name drugs. All generic drugs are approved by the FDA and must be manufactured in accordance with the same good manufacturing practices as brand-name drugs.
Do Generic Drugs Contain Different Ingredients?
No, generic drugs contain the same active ingredients as their brand-name equivalents. The inactive ingredients in generic drugs may differ from their brand-name equivalents, but these ingredients are typically not related to the therapeutic effects of the drug.
Are Generic Drugs Available for All Medications?
No, generic drugs are not available for all medications. Generally, generic drugs are only available for medications that have been approved by the FDA for more than five years. Additionally, some medications may be protected by patents or other exclusivity rights, which can prevent the approval of generic versions of the drug.
Generic Drugs
In conclusion, generic drugs are an important part of our healthcare system. They provide us with an affordable alternative to brand name drugs, while still providing us with the same effectiveness and safety. Generic drugs can also help to reduce healthcare costs for individuals, as well as for insurance companies and the government. With the increasing cost of healthcare, generic drugs offer an important way to manage costs and ensure access to safe, effective medications.

