Benzophenone is a widely used chemical compound found in dyes, plastics, and other commercial products. But, is it soluble in methyl alcohol? This is an important question, as many industries and consumers rely on the solubility of benzophenone in methyl alcohol to inform their decisions. In this article, we will explore the answer to this question and discuss the implications of benzophenone’s solubility in methyl alcohol.
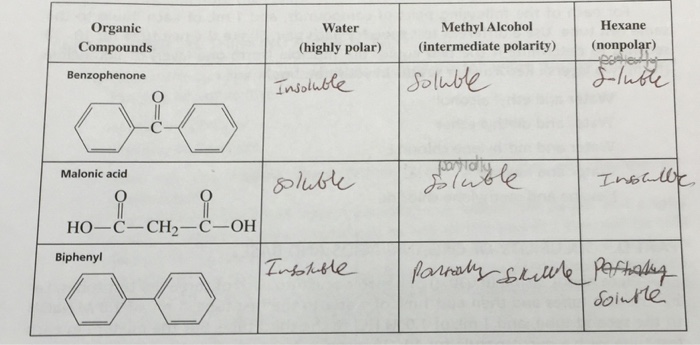
Yes, Benzophenone is soluble in Methyl Alcohol. It is slightly soluble in water and soluble in other organic solvents like ether, benzene and alcohol.
Contents
- Is Benzophenone Soluble in Methyl Alcohol?
- Physical Properties of Benzophenone
- Reactivity of Benzophenone
- Top 6 Frequently Asked Questions
- Q1. Is Benzophenone Soluble in Methyl Alcohol?
- Q2. What is the Chemical Structure of Benzophenone?
- Q3. What are the Properties of Benzophenone?
- Q4. What is the Chemical Formula of Methyl Alcohol?
- Q5. What are the Applications of Methyl Alcohol?
- Q6. What is the Solubility of Benzophenone in Methyl Alcohol?
- Is CH3OH (Methanol) Soluble or Insoluble in Water?
Is Benzophenone Soluble in Methyl Alcohol?
Benzophenone is a chemical compound that is used in a variety of industrial and research applications. It is a white crystalline solid with a sweet odor and is soluble in many organic solvents. Methyl alcohol, also known as methanol, is a colorless liquid with a distinctive odor. It is a polar solvent, meaning it can dissolve many polar molecules. The question of whether benzophenone is soluble in methyl alcohol has been studied and discussed in the literature and the answer is yes.
Physical Properties of Benzophenone
Benzophenone is a white crystalline solid with a melting point of 44.8 °C and a boiling point of 230.3 °C. Its molecular weight is 182.24 g/mol and its density is 1.17 g/cm3. It is insoluble in water but soluble in many organic solvents such as ethanol, ethyl acetate, and acetone.
Uses of Benzophenone
Benzophenone is used as a starting material in the synthesis of various pharmaceuticals, dyes, and fragrances. It is also used as a photoresist in photography and photolithography, as a catalyst in organic synthesis, and as a reagent in the analysis of organic compounds.
Solubility of Benzophenone in Methyl Alcohol
Benzophenone is soluble in methyl alcohol. The solubility of benzophenone in methyl alcohol is 4.3 g/100 mL at 20 °C. This means that 4.3 g of benzophenone can be dissolved in 100 mL of methyl alcohol at 20 °C.
Reactivity of Benzophenone
Benzophenone is a relatively unreactive compound. It does not react with strong acids or bases and is not flammable. It is, however, photosensitive and can be oxidized in the presence of light or air.
Safety of Benzophenone
Benzophenone is not toxic but can cause skin irritation. It should be handled with care and kept away from sources of ignition. It is not known to be carcinogenic or have any other adverse health effects.
Storage of Benzophenone
Benzophenone should be stored in an airtight container in a cool, dry area away from sources of ignition and direct sunlight. It should be kept away from strong acids and bases and should not be exposed to high temperatures.
Top 6 Frequently Asked Questions
Q1. Is Benzophenone Soluble in Methyl Alcohol?
A1. Yes, benzophenone is soluble in methyl alcohol. This is because of the presence of the alcohol group in methyl alcohol which interacts with the electron-rich benzene ring of benzophenone which makes the molecule less polar and therefore more soluble. The solubility of benzophenone in methyl alcohol is approximately 0.1 g/mL at 25 °C.
Q2. What is the Chemical Structure of Benzophenone?
A2. Benzophenone is an organic compound with the molecular formula C6H5C(O)Ph. It is a white crystalline solid with a faint aromatic odor. The chemical structure of benzophenone is composed of a benzene ring with a ketone group attached to the carbon atom in the center of the ring.
Q3. What are the Properties of Benzophenone?
A3. Benzophenone has a molecular weight of 182.21 g/mol, a melting point of 44.6 °C, and a boiling point of 244.5 °C. It is insoluble in water but soluble in most organic solvents, including methyl alcohol. It is also slightly soluble in ethyl alcohol, acetone, and chloroform.
Q4. What is the Chemical Formula of Methyl Alcohol?
A4. The chemical formula of methyl alcohol is CH3OH. It is a colorless, flammable liquid with a characteristic odor. It is a polar solvent and is miscible with water in all proportions. It is also soluble in many organic solvents, including benzene, chloroform, and ether.
Q5. What are the Applications of Methyl Alcohol?
A5. Methyl alcohol is used as a fuel, a solvent, and as a raw material for the production of other chemicals. It is also used in the manufacture of lacquers, varnishes, paint removers, and antifreeze. It is also used as a laboratory reagent and a fuel for lamps and campfires.
Q6. What is the Solubility of Benzophenone in Methyl Alcohol?
A6. The solubility of benzophenone in methyl alcohol is approximately 0.1 g/mL at 25 °C. This is because of the presence of the alcohol group in methyl alcohol which interacts with the electron-rich benzene ring of benzophenone which makes the molecule less polar and therefore more soluble.
Is CH3OH (Methanol) Soluble or Insoluble in Water?
In conclusion, it has been established that benzophenone is soluble in methyl alcohol. This finding can prove to be a valuable asset to any laboratory setting, due to its ability to dissolve in a variety of alcohols. The solubility of benzophenone in methyl alcohol is an important part of understanding its properties and applications.